

TRILOGY (COMBINED) SCIENCE
The following information shows the topics covered as part of the Trilogy (Combined) GCSE Science course. All three subjects are taught as part of Trilogy but the topics are often shorter than the versions found in the Separate Sciences. The table of information has been taken from the official AQA GCSE Trilogy Course Specifications. The table below is split in BIOLOGY, CHEMISTRY and PHYSICS

SUBTOPIC
CONTENT
Cell structure
Eukaryotes and prokaryotes
Animal and plant cells
Cell specialisation
Cell differentiation
Microscopy
Animal and plant cells
Cell specialisation
Cell differentiation
Microscopy
Cell division
Chromosomes
Mitosis and the cell cycle
Stem cells
Mitosis and the cell cycle
Stem cells
Transport in cells
Diffusion
Osmosis
Active transport
Osmosis
Active transport
Principles of organisation
Animal tissues, organs and organ systems
The human digestive system
The heart and blood vessels
Blood
Coronary heart disease: a non communicable disease
Health issues
The effect of lifestyle on some non-communicable diseases
Cancer
The heart and blood vessels
Blood
Coronary heart disease: a non communicable disease
Health issues
The effect of lifestyle on some non-
Plant tissues, organs and systems
Plant tissues
Plant organ system
Plant organ system
Communicable diseases
Communicable (infectious) diseases
Viral diseases
Bacterial diseases
Fungal diseases
Protist diseases
Human defence systems
Vaccination
Antibiotics and painkillers
Discovery and development of drugs
Viral diseases
Bacterial diseases
Fungal diseases
Protist diseases
Human defence systems
Vaccination
Antibiotics and painkillers
Discovery and development of drugs
Photosynthesis
Photosynthetic reaction
Rate of photosynthesis
Uses of glucose from photosynthesis
Rate of photosynthesis
Uses of glucose from photosynthesis
Respiration
Aerobic and anaerobic respiration
Response to exercise
Metabolism
Response to exercise
Metabolism
Homeostasis
The human nervous system
Structure and function
Hormonal coordination in humans
Human endocrine system
Control of blood glucose concentration
Hormones in human reproduction
Contraception
The use of hormones to treat infertility (Higher Tier only)
Negative feedback (Higher Tier only)
Control of blood glucose concentration
Hormones in human reproduction
Contraception
The use of hormones to treat infertility (Higher Tier only)
Negative feedback (Higher Tier only)
Reproduction
Sexual and asexual reproduction
Meiosis
DNA and the genome
Genetic inheritance
Inherited disorders
Sex determination
Meiosis
DNA and the genome
Genetic inheritance
Inherited disorders
Sex determination
Variation and evolution
Variation
Evolution
Selective breeding
Genetic engineering
Evolution
Selective breeding
Genetic engineering
The development of understanding of genetics and evolution
Evidence for evolution
Fossils
Extinction
Resistance bacterial
Fossils
Extinction
Resistance bacterial
Classification of living organisms
Adaptations, interdependence and competition
Communities
Abiotic factors
Biotic factors
Adaptations
Abiotic factors
Biotic factors
Adaptations
Organisation of an ecosystem
Levels of organisation
How materials are cycled
How materials are cycled
Biodiversity and the effect of human interaction on ecosystems
Biodiversity
Waste management
Land use
Deforestation
Global warming
Maintaining biodiversity
Waste management
Land use
Deforestation
Global warming
Maintaining biodiversity
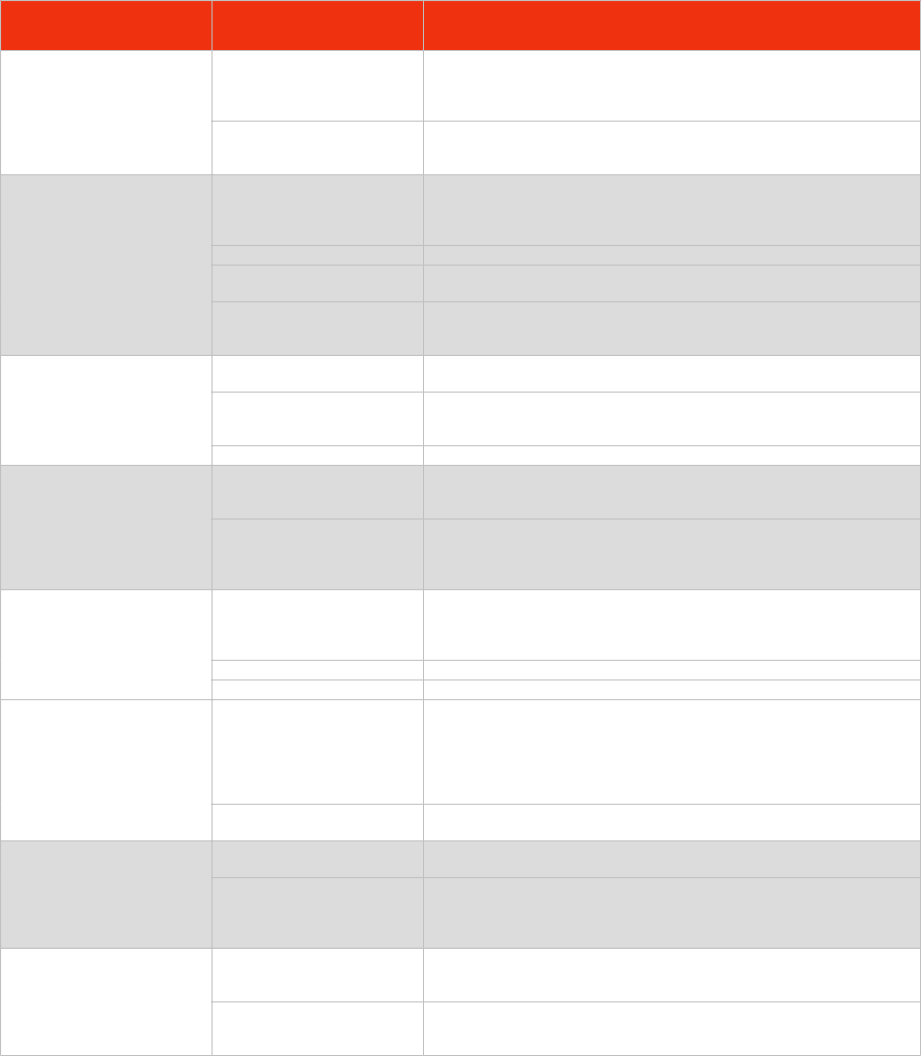
SUBTOPIC
CONTENT
Energy changes in a system, and the ways energy is stored before and after such changes
Energy stores and systems
Changes in energy
Energy changes in systems
Power
Changes in energy
Energy changes in systems
Power
Conservation and dissipation of energy
Energy transfers in a system
Efficiency
National and global resources
Efficiency
National and global resources
Current, potential difference and resistance
Standard circuit diagram symbols
Electrical charge and current
Current, resistance and potential difference
Resistors
Electrical charge and current
Current, resistance and potential difference
Resistors
Series and parallel circuits
Domestic uses and safety
Direct and alternating potential difference
Main electricity
Main electricity
Energy transfers
Power
Energy transfers in everyday appliances
The National Grid
Energy transfers in everyday appliances
The National Grid
Changes of state and the particle model
Density of materials
Change of state
Change of state
Internal energy and energy transfers
Internal energy
Temperature changes in a system and specific heat capacity
Changes of heat and specific latent heat
Temperature changes in a system and specific heat capacity
Changes of heat and specific latent heat
Particle model and pressure
Particle motion in gases
Atoms and isotopes
The structure of an atom
Mass number, atomic number and isotopes
The development of the model of the atom (common content with chemistry)
Mass number, atomic number and isotopes
The development of the model of the atom (common content with chemistry)
Atoms and nuclear radiation
Radioactive decay and nuclear radiation
Nuclear equations
Half-lives and the random nature of radioactive decay
Radioactive contamination
Nuclear equations
Half-
Forces and their interactions
Scalar and vector quantities
Contact and non-contact forces
Gravity
Resultant forces
Contact and non-
Resultant forces
Work done and energy transfer
Forces and elasticity
Forces and motion
Describing motion along a line: Distance and displacement; Speed; Velocity; The distance-time relationship; Acceleration
Forces acceleration and Newton’s Laws of motion: Newton’s First Law; Newton’s Second Law; Newton’s Third Law
Forces and braking: Stopping distance; Reaction time’; Factors affecting braking distance 1; Factors affecting braking distance 2
Forces and braking: Stopping distance; Reaction time’; Factors affecting braking distance 1; Factors affecting braking distance 2
Momentum (Higher Tier only)
Momentum is a property of moving objects
Conservation of momentum
Conservation of momentum
Waves in air, fluids and solids
Transverse and longitudinal waves
Properties of a wave
Properties of a wave
Electromagnetic waves
Types of electromagnetic waves
Properties of electromagnetic waves 1
Properties of electromagnetic waves 2
Use and applications of electromagnetic waves
Properties of electromagnetic waves 1
Properties of electromagnetic waves 2
Use and applications of electromagnetic waves
Permanent and induced magnetism, magnetic forces and fields
Poles of a magnet
Magnetic fields
Magnetic fields
The motor effect
Electromagnetism
Fleming’s left-hand rule (Higher Tier only)
Electric motors (Higher Tier only)
Fleming’s left-
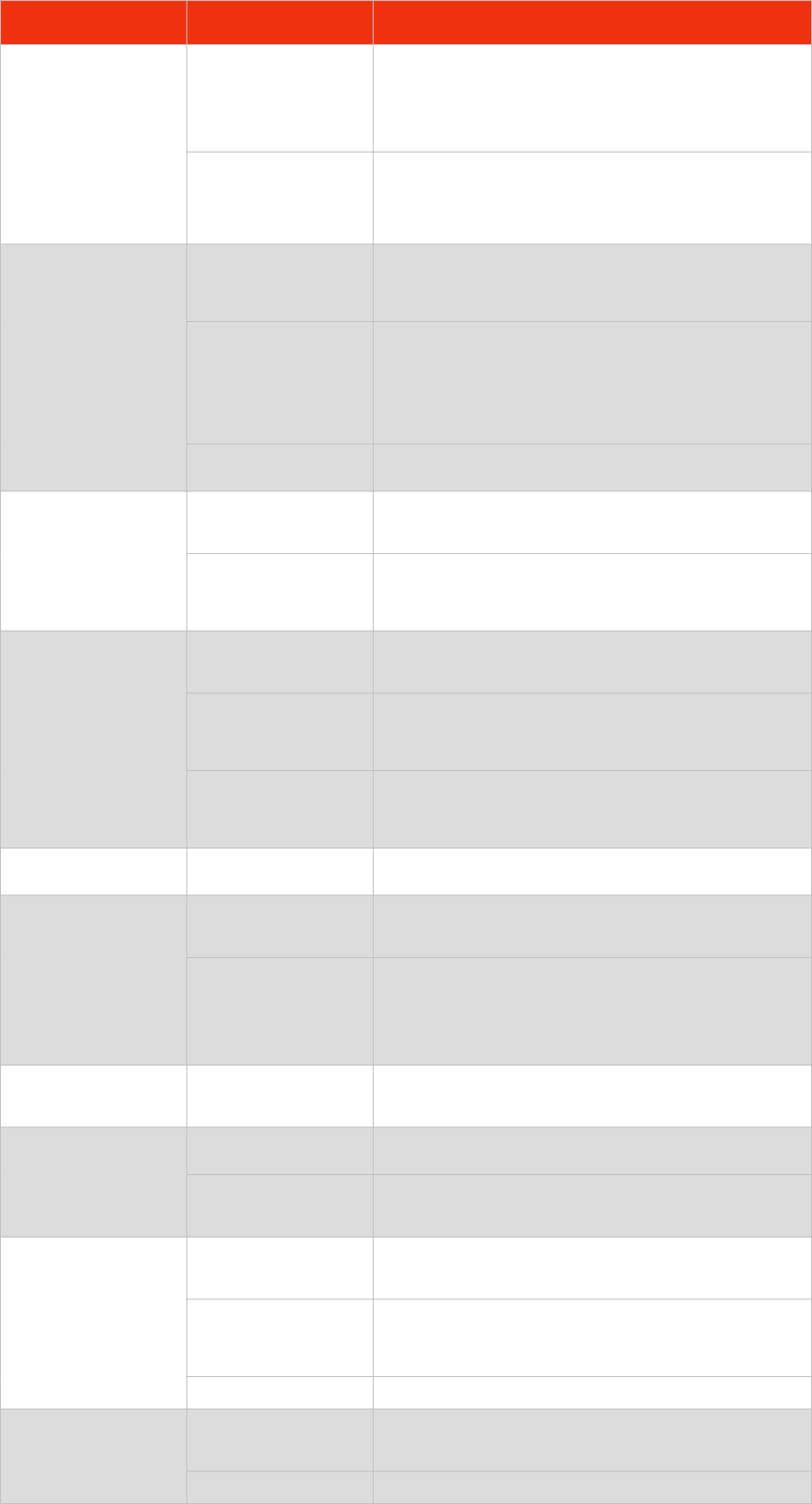
SUBTOPIC
CONTENT
A simple model of the atom, symbols, relative atomic mass, electronic charge and isotopes
Atoms, elements and compounds
Mixtures
The development of the model of the atom (common content with physics P4)
Relative electrical charges of subatomic particles
Size and mass of atoms
Relative atomic mass
Electronic structure
Mixtures
The development of the model of the atom (common content with physics P4)
Relative electrical charges of subatomic particles
Size and mass of atoms
Relative atomic mass
Electronic structure
The periodic table
The periodic table
Development of the periodic table
Metals and non-metals
Group 0
Group 1
Group 7
Development of the periodic table
Metals and non-
Group 1
Group 7
Chemical bonds, ionic, covalent and metallic
Chemical bonds
Ionic Bonding
Ionic compounds
Covalent bonding
Metallic bonding
Ionic Bonding
Ionic compounds
Covalent bonding
Metallic bonding
How bonding and structure are related to the properties of substances
The three states of matter
State symbols
Properties of ionic compounds
Properties of small molecules
Polymers
Giant covalent structures
Properties of metals and alloys
Metals as conductors
State symbols
Properties of ionic compounds
Properties of small molecules
Polymers
Giant covalent structures
Properties of metals and alloys
Metals as conductors
Structure and bonding of carbon
Diamond
Graphite
Graphenes and fullerenes
Graphite
Graphenes and fullerenes
Chemical measurements, conservation of mass and the quantitative interpretation of chemical equations
Conservation of mass and balanced chemical equations
Relative formula mass
Mass changes when the reactant or product is a gas
Chemical measurements
Relative formula mass
Mass changes when the reactant or product is a gas
Chemical measurements
Use of amount of substance in relation to masses of pure substances
Moles (Higher Tier only)
Amount of substances in equations (Higher Tier only)
Using moles to balance equations (Higher Tier only)
Limiting reactants (Higher Tier only)
Concentrations of solution
Amount of substances in equations (Higher Tier only)
Using moles to balance equations (Higher Tier only)
Limiting reactants (Higher Tier only)
Concentrations of solution
Reactivity of metals
Metal oxides
The reactivity series
Extraction of metals and reduction
Oxidation and reduction in terms of electrons (Higher Tier only)
The reactivity series
Extraction of metals and reduction
Oxidation and reduction in terms of electrons (Higher Tier only)
Reactions of acids
Reactions of acids with metals
Neutralisation of acids and salt production
Soluble salts
The ph scale and neutralisation
Strong and weak acids (Higher Tier only)
Neutralisation of acids and salt production
Soluble salts
The ph scale and neutralisation
Strong and weak acids (Higher Tier only)
Electrolysis
The process of electrolysis
Electrolysis and molten ionic compounds
Using electrolysis to extract metals
Electrolysis of aqueous solutions
Representation of reactions at electrodes as half equations (Higher Tier only)
Electrolysis and molten ionic compounds
Using electrolysis to extract metals
Electrolysis of aqueous solutions
Representation of reactions at electrodes as half equations (Higher Tier only)
Exothermic and endothermic reactions
Energy transfer during exothermic and endothermic reactions
Reaction profiles
The energy change of reactions (Higher Tier only)
Reaction profiles
The energy change of reactions (Higher Tier only)
Rate of reaction
Calculating rates of reactions
Factors which affect the rates of chemical reactions
Collision theory and activation theory
Catalysts
Factors which affect the rates of chemical reactions
Collision theory and activation theory
Catalysts
Reversible reactions and dynamic equilibrium
Reversible reactions
Energy changes and reversible reactions
Equilibrium
The effect of changing conditions on equilibrium (Higher Tier only)
The effect of changing concentration (Higher Tier only)
The effect of temperature changes on equilibrium (Higher Tier only)
The effect of pressure changes on equilibrium (Higher Tier only)
Energy changes and reversible reactions
Equilibrium
The effect of changing conditions on equilibrium (Higher Tier only)
The effect of changing concentration (Higher Tier only)
The effect of temperature changes on equilibrium (Higher Tier only)
The effect of pressure changes on equilibrium (Higher Tier only)
Carbon compounds as fuels and feedstock
Crude oil, hydrocarbons and alkanes
Fractional distillation and petrochemicals
Properties of hydrocarbons
Cracking and alkenes
Fractional distillation and petrochemicals
Properties of hydrocarbons
Cracking and alkenes
Purity, formulations and chromatography
Pure substances
Formulations
Chromatography
Formulations
Chromatography
Identification of common gases
Test for hydrogen
Test for oxygen
Test for carbon dioxide
Test for chlorine
Test for oxygen
Test for carbon dioxide
Test for chlorine
The composition and evolution of the Earth’s composition
The proportions of different gases in the atmosphere
The Earth’s early atmosphere
How oxygen increased
How carbon dioxide decreased
The Earth’s early atmosphere
How oxygen increased
How carbon dioxide decreased
Carbon dioxide and methane as greenhouse gases
Greenhouse gases
Human activities which contribute to an increase in greenhouse gases in the atmosphere
Global climate change
The carbon footprint and its reduction
Human activities which contribute to an increase in greenhouse gases in the atmosphere
Global climate change
The carbon footprint and its reduction
Common atmospheric pollutants and their sources
Atmospheric pollutants from fuels
Properties and effects of atmospheric pollutants
Properties and effects of atmospheric pollutants
Using the Earth’s resources and obtaining portable water
Using the Earth’s resources and sustainable development
Portable water
Waste water management
Alternative methods of extracting metals (Higher Tier only)
Portable water
Waste water management
Alternative methods of extracting metals (Higher Tier only)
Life cycle assessment and recycling
Life cycle assessment
Ways of reducing the the use of the resources
Ways of reducing the the use of the resources
TOP
TOP
COMBINED
BIOLOGY
CHEMISTRY
PHYSICS
OVERVIEW
TOP